Keratoconus; A Big Word With A Big Impact On Vision
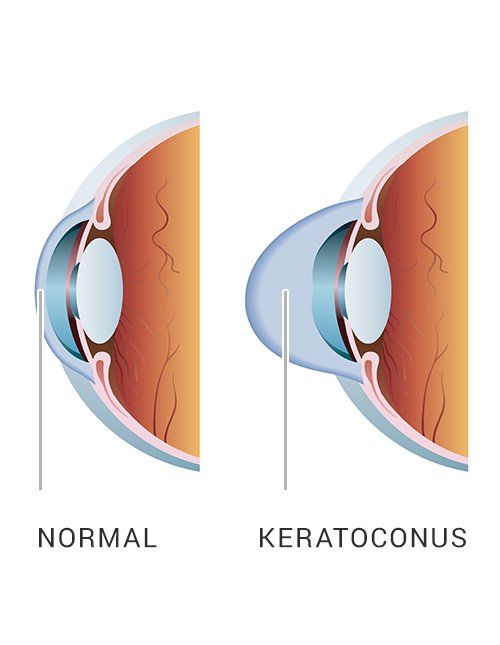
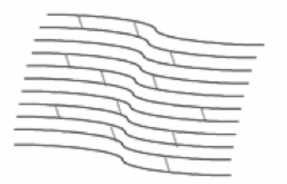
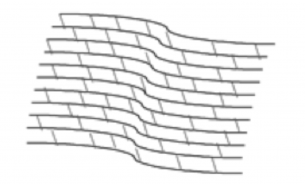
Keratoconus, sometimes referred to as “KC”, is a progressive eye condition in which the cornea weakens and thins over time causing the typically round, dome-shaped cornea to develop a cone-like bulge that produces optical irregularities affecting vision. Keratoconus typically appears in individuals who are in their late teens or early twenties and affects an estimated one in two thousand persons in the United States. Early symptoms include; blurring or distortion of vision, increased eye rubbing, increased sensitivity to light and frequent changes in prescription or the inability to effectively correct vision with prescription glasses. The exact cause for keratoconus is unknown though some research indicates that a hereditary component may have an impact on developing the condition.
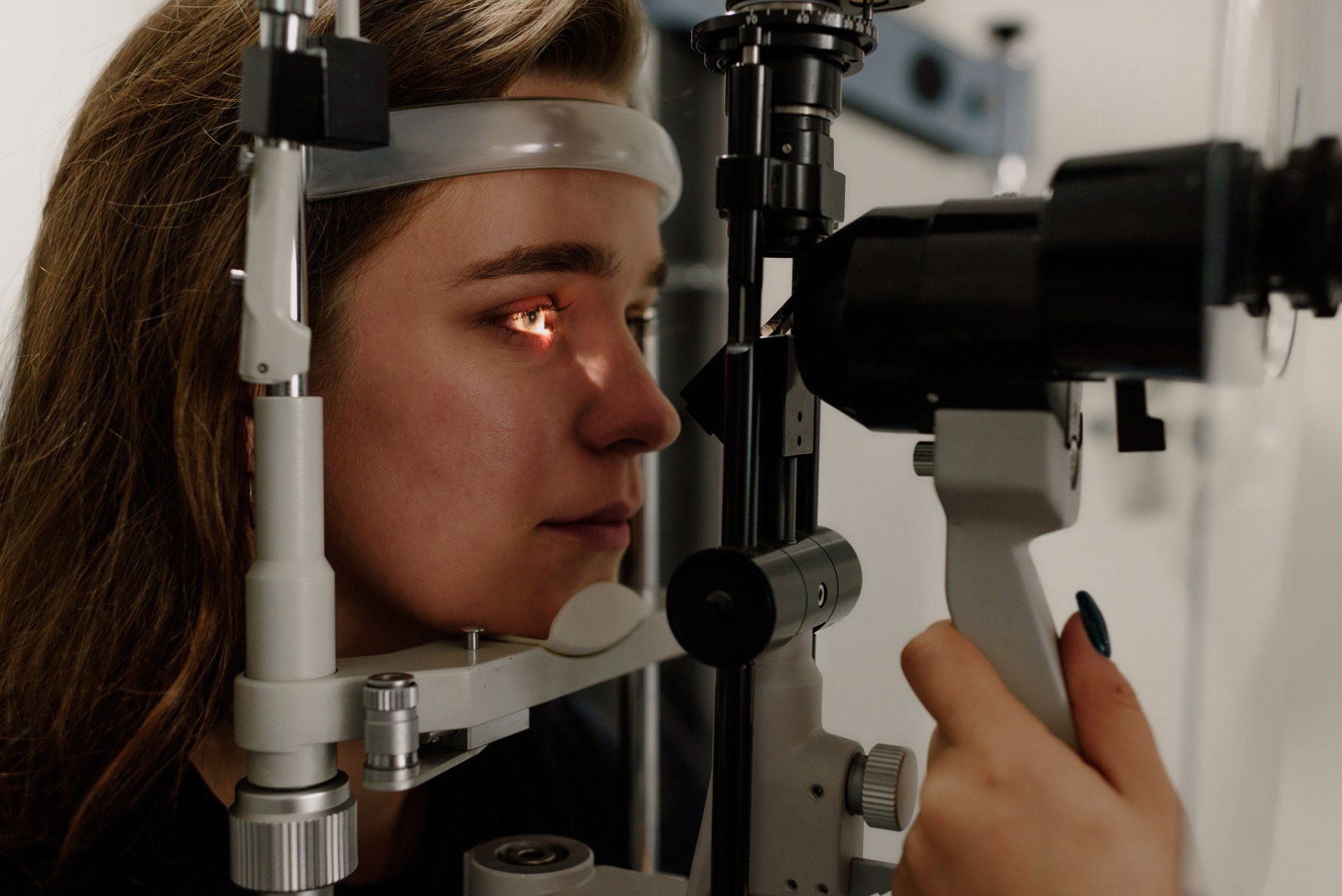
The cornea is responsible for focusing most of the light that comes into the eye. Therefore, abnormalities of the cornea, such as keratoconus, can have a major impact on how an individual sees the world. Progressive keratoconus can result in significant visual loss and in severe cases, can lead to the need for corneal transplant. Early detection and diagnosis are critical in the treatment of keratoconus to prevent the disease from progressing and worsening vision.
Up until recently, there weren’t many treatment options available for progressive keratoconus. While wearing rigid gas permeable (RGP) contact lenses is an available treatment option, because of the cornea’s irregular shape, these lenses can be very challenging to fit. This process often requires a great deal of time and patience.
Corneal Crosslinking is the only FDA approved therapeutic option to limit the progression of keratoconus. Crosslinking combines the use of ultra-violet (UV) light and riboflavin (vitamin B2) eye drops. The procedure works by creating new corneal collagen cross-links, which results in a shortening and thickening of collagen fibrils that leads to stiffening of the cornea. Crosslinking, which has been performed in Europe since 2003 and Canada since 2008, is considered the standard of care around the world for treating keratoconus.
Talley Eye Institute is the first practice in the Tri-State area that is treating keratoconus using the FDA approved Corneal Crosslinking procedure. Dwight Silvera, MD, FRCSC, DABO, performs Corneal Crosslinking and began performing the procedure in Canada in 2009. If you or a loved one have been diagnosed with keratoconus or are at risk (family history), it’s imperative to find a qualified ophthalmologist to manage and treat the condition. Call today if you’d like more information or to schedule an evaluation with Dr. Silvera, 812-424-2020.